Your Dedicated Platform for ADC Analytical Development to Foster New & Existing Relationships with Key Decision Makers Seeking Your Expertise
The 5th ADC Analytical Development Summit returns to Boston uniting 80+ senior analytical scientists, CMC leaders, and quality heads from big pharma and innovative biotechs. As the industry’s definitive forum hyper-focused on ADC analytical science, this event is designed to tackle the most complex challenges in characterizing, controlling, and filing for the next generation of antibody-drug conjugates.
Through deep engagement with the ADC analytical community, we’ve identified their critical pain points, including:
- Controlling and Understanding Impurities for linkers and payloads
- Implementing Advanced, Robust Analytical Methods for ADCs & AOCs
- Ensuring Reliable Method Transfer to Quality Control labs and CDMOs
- Developing Bioanalytical Assays with strict CRO timelines
- Navigating Evolving Regulatory Expectations in a "case-by-case" environment
Partner with us in 2026 to elevate your brand as the go-to analytical partner and secure direct access to the very specific audience of analytical senior decision-makers and technical experts from companies like AbbVie, Pfizer, AstraZeneca, Merck, and Bristol Myers Squibb, all actively seeking the specialized expertise and reliable solutions needed to de-risk their analytical development and accelerate ADC therapies to market.

Why Should You Prioritize Partnering with Us:
Raise Your Brand Awareness
Showcase your solutions to the key opinion leaders and decision-makers who rely on specialized partners to solve critical challenges in advanced DAR analysis, impurity control, potency assay development, and robust method transfer for next-generation ADCs.
Generate Commercial Opportunities
Intentionally curated to foster high-value connections between solution providers and the analytical leaders who need them most. With an intimate environment, you’ll engage in meaningful dialogues with senior scientists and directors.
Distinguish Your Solutions
Stand out by demonstrating your expertise in overcoming the unique hurdles of ADC analytical development, including:
- Advanced Characterization: Highlight capabilities in native MS, 2D-LC/MS, capillary electrophoresis, and platform methods to characterize bispecifics, dual-payload conjugates, and novel modalities.
- Impurity & Stability Mastery: Showcase specialized tools and expertise for identifying novel degradation products, conducting forced degradation studies, and setting scientifically justified specifications.
- Bioanalytical Excellence: Demonstrate your proficiency in developing and transferring complex cell-based potency assays, PK/PD studies, and stability-indicating methods.
- CMC & Regulatory Support: Position your services in analytical method validation, comparability studies, and constructing compelling regulatory narratives for novel ADC attributes.
Powering Innovation in ADC Analytical Development
Attendees from 80+ leading biopharma and biotech companies are seeking partners with expertise in late-stage characterization, novel modality control, and seamless tech transfer. Whether your focus is advanced instrumentation, method development, bioanalytical services, or regulatory strategy, partnering with us connects you directly with senior decision-makers.
1. CDMOs
ADC developers urgently require partners with proven expertise in GMP manufacturing, conjugation, and scale-up. Showcase how analytical method development, process-related impurity control, and reliable tech transfer capabilities bridge the gap between innovative biotech’s and robust, scalable production.
2. Analytical CROs
Developers need services in advanced chromatography (HIC, SEC, CEX), mass spectrometry (LC-MS, native MS), and forced degradation studies for deep product understanding and successful regulatory submissions.
3. Bioanalytical CROs
Expertise complex cell-based potency assays, PK/PD studies, and immunogenicity testing (ADA) is essential to provide critical biological data that links analytical attributes to clinical efficacy.
4. Equipment & Software Providers
Higher resolution, automation, and data intelligence are required for advanced HPLC/UPLC, capillary electrophoresis systems, integrated stability platforms, and analytical software to transforms complex datasets.
Hear What Our Past Sponsors Have to Say

Small size facilitated easy discussions with fellow attendees
Veranova

My experience was truly enjoyable due to the engaging atmosphere, which made it easy to connect with other people and have interesting conversations not just from the sessions, but also from casual chats during the breaks. I also appreciated the variety of topic covered throughout the event.
What stood out the most was the quality of the presentations, which were very well structured, and I especially appreciated the roundtable moments, which encouraged meaningful discussion and knowledge sharing.
BSP Pharmaceuticals
Attending Companies Include




Available Inventory
We have a range of inventory from standard booth and speaking slots to more bespoke deliverable such as badges, exclusive dinners and tours to help elevate your brand.

Presenting Slots
Get involved in data-driven/ case study-led presentations to position yourself as a thought-leader.
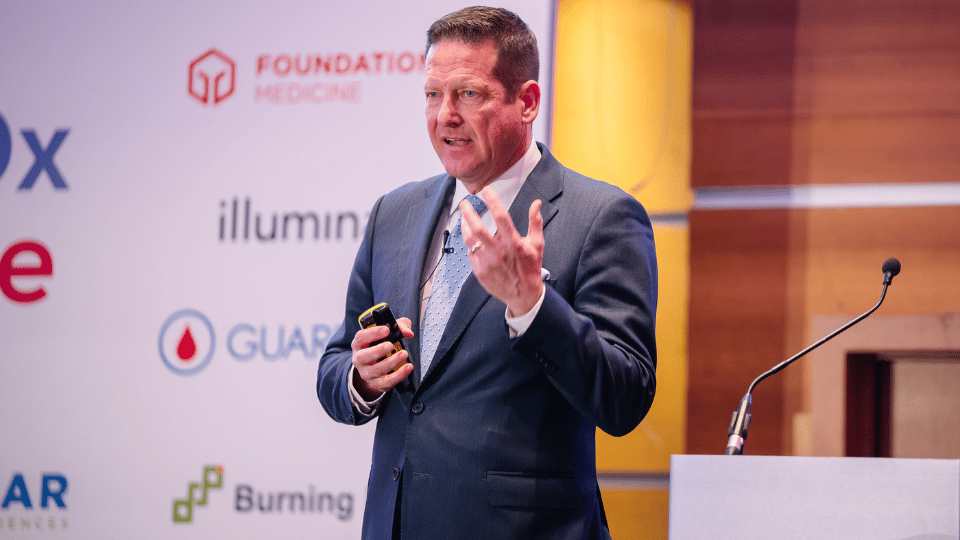
Leading Innovation
Join expert panels to shape forward-thinking discussions.

Full Delegate List
Gain exclusive access to the Full Delegate List to plan your conversations in advance.

Exhibiting
Raise brand awareness and answer questions from prospective customers.

Bespoke Hosting
Host breakfast, lunches, dinners for drinks receptions to have exclusive time with your target audience.

Champion the Industry
Be part of the ADC analytical community and position yourself as an advocate for innovation and achievements in the field.
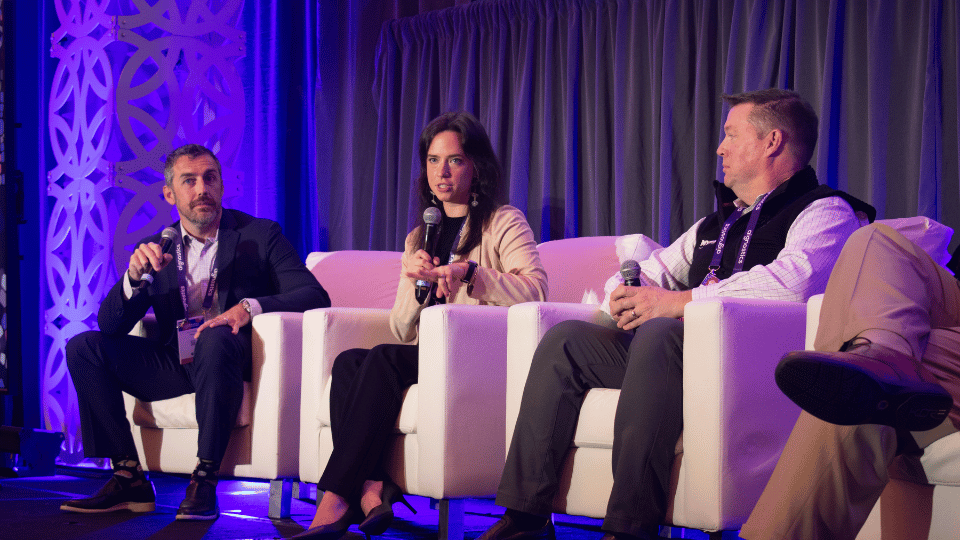
Branding
On-site and website branding to highlight your company at the forefront of the ADC analytical field.

Networking
Engage in 1-2-1 conversations with industry or be part of the organized networking options to generate commercial collaborations.
Get in Touch
Take advantage of our bespoke sponsorship opportunities to achieve your commercial goals. Email us if you would like to get involved and discuss a bespoke package suited to your needs.

George Shrimpton
Partnerships Director



