Explore Advances in ADC Characterization
Explore advanced analytical techniques like LC-MS, SEC, and HIC to gain insights into ADC quality attributes. These methods enable precise analysis of DAR distributions, structural integrity, and stability, ensuring consistent product quality. Leveraging these approaches enhances ADC characterization, efficacy, and safety, leading to successful clinical outcomes.
Optimize Potency & Cell-Based Assays
Refine your potency and cell-based assay strategies to enhance sensitivity and reproducibility. Learn to design standardized protocols that align with GMP standards, reduce assay variability, and deliver reliable data for ADC development. These improvements are crucial for ensuring accurate potency readouts and confidently supporting regulatory submissions.
Stay Ahead with Cutting-Edge Analytical Strategies
Gain expertise in developing phase-appropriate analytical methods for ADC development, from preclinical research to BLA submission. Discover techniques to enhance data accuracy, streamline regulatory approvals, and ensure commercialization readiness. Fine-tuning your approaches can significantly impact the efficiency and success of your ADC program.
Master Regulatory Insights Across Regions
Understand the evolving global regulatory landscape for ADCs, focusing on EU and US guidelines. Learn to align your analytical strategies with regional expectations to ensure compliance and accelerate approval timelines. Mastering these regulatory nuances will help you navigate the complexities of global ADC development with confidence.
Connect with Industry Leaders & Innovators
Engage with analytical development experts from leading biotech and pharmaceutical companies through workshops, roundtables, and case study discussions. Exchange insights and collaborate on solutions to overcome key challenges in ADC analytics. Build connections that drive innovation and success in your ADC programs.
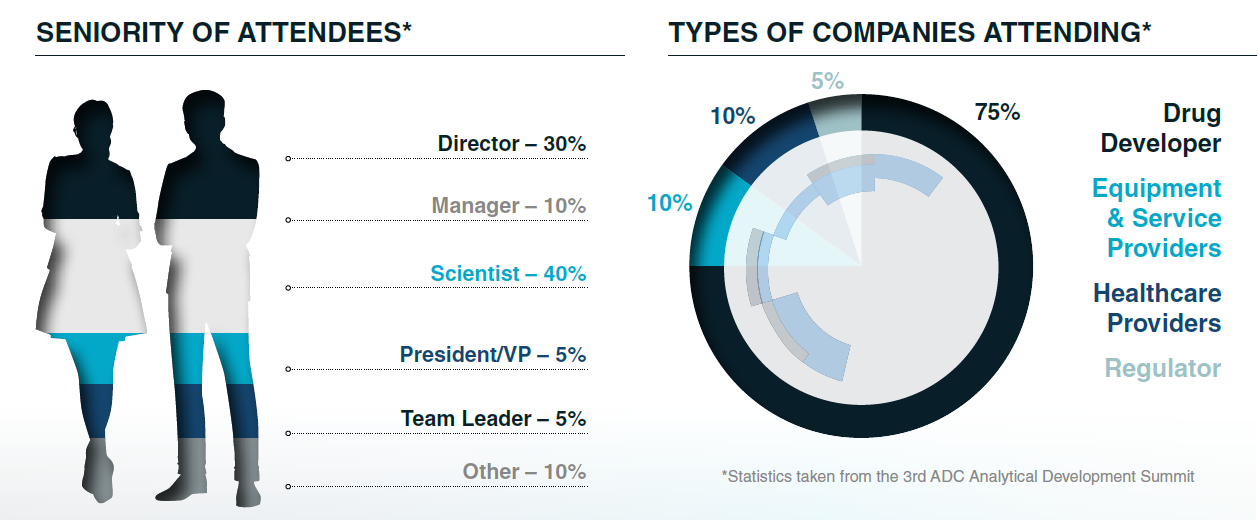
Who Attended?
Over 120 professionals from analytical development, CMC, quality control, and technical operations